66 |
Oilfield Technology
June
2015
flooding operations operate with an oil cut of less than 20%, because
the water injected is unable to displace the crude oil trapped in
the tight carbonate rocks. Furthermore, the huge volumes of water
produced need to be treated and recycled; the higher the water cut,
the higher the operating costs. Adding surfactant formulations to
the injected water has the potential to improve the water flooding
process significantly by favourably affecting the oil cut, and the
overall oil recovery from the reservoirs.
School of rock
Carbonate reservoirs are a challenging porous rock system with pore
networks spanning a wide gamut of length scales: from the tight low
permeability matrix with length scales less than a micron, to vugs, or
connected porous networks on millimetre length scales created over
decades of water flooding, to high permeability fracture networks,
which can be a millimetre wide with length span of a few hundred
meters. The high permeability zones are easy to flow through and
are well swept by water, allowing ‘easy’ oil residing in these zones
to be recovered early on during the water flooding process. The
‘difficult’ oil trapped in tight pores is inaccessible to the water flow
path and is left behind. Furthermore, since the carbonate rocks
have a preferential affinity towards oil, water imbibition into the
tight carbonate matrix becomes very challenging. Thus, oil recovery
factors by water flooding in carbonate formations have remained low
(typically less than 30%) primarily due to:
Ì
Significant permeability contrasts leading to poor sweep
efficiency.
Ì
Significant amounts of oil trapped in tight low permeability
pores.
Ì
Low imbibition of water in tight pores.
Ì
Heterogeneous reservoir rock properties.
To improve the oil recovery factor, specially tailored chemical
formulations can be added during the water flooding process to allow
water to sweep the tight pores, effectively recovering the ‘difficult’ oil
left behind.
Enhancedwater flooding: thenew
frontier
Since carbonate reservoirs have low oil
recovery due to their preferential affinity to
oil (oil‑wet rock surface) and the trapping of
oil in tight pores, a viable method to release
the trapped oil is through the addition of
surfactants that enhance imbibition of water
in tight pores by capillary action. Capillary
action is the primary mechanism behind many
natural processes, like the rise of water in tall
trees through long slender pathways. The
phenomena of enhancing water imbibition into
tight pores and displacing oil trapped in the
pores by capillary action depends primarily on
the affinity of the rock surface (wettability) to
the imbibing medium (water), the interfacial
tension (IFT) between the imbibing fluid
(water) and the displaced fluid (oil), and the
pore diameter. The preferential oil wetness of
the carbonates and the interfacial resistance
between oil and water adversely affect the
displacement of oil through the tight pores.
A certain class of specially designed
surfactant formulations can significantly
enhance the capillary imbibition of water by
altering the affinity of carbonate rock surface
from oil (oil‑wet) to water (water‑wet). Through
application of such formulations, oil attached
to the rock is released and the imbibition of
water into tight matrix is enhanced. This results
in the further release of additional oil, while
allowing more water to be imbibed into the
rock matrix. An illustration of this concept is
shown in Figure 1. Furthermore, if more water
can be injected while keeping the injection flow
pressure constant, more fluid can be produced
without any additional energy input. The
application of such surfactant formulations
must be economically viable. Therefore, the
surfactant formulation must be an effective
wettability altering agent at low dosages.
The phenomena of wettability alteration
of rock surfaces can also be achieved by
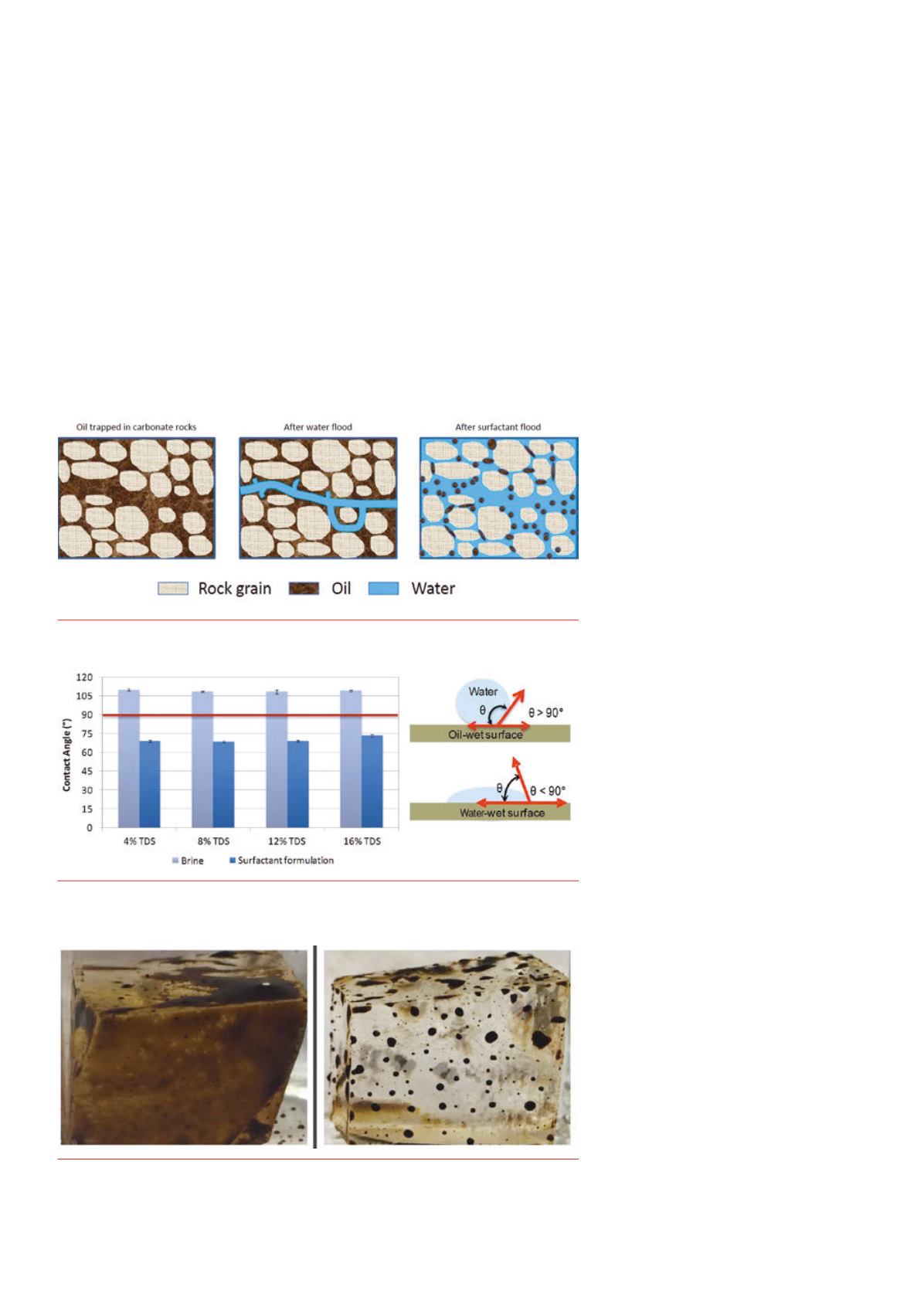
Figure 1.
An illustration of enhanced imbibition of water in oil‑wet carbonate rockmatrix using
surfactant formulation.
Figure 2.
Contact angle results for brine ona hydrophobic surface before andafter addition of
surfactant formulation (left); and schematic illustration of the change in contact angle ona surface
after wettability alteration fromoil‑wet towater‑wet state (right).
Figure 3.
Visuals of oil–wet calciteplates exposed tobrine (left), and surfactant formulation inbrine
(right). The calcite plate on the right shows the oil has been etched out and the oil‑wet surface has
beenmodified to becomemorewater‑wet.


